|
钴基催化剂正在水中将脂肪酸和麻风树油间接选择性氢化为脂肪醇
Energy & Fuels
(
IF
5.2
)
Pub Date : 2018-07-03 00:00:00
, DOI:
10.1021/acs.energyfuels.8b01114
Wenda Jia
1
,
Guangyue Xu
1
,
Xiaohao Liu
1
,
Feng Zhou
2
,
HuiVia Ma
2
,
Ying Zhang
1
,
Yao Fu
1
Affiliation
iChEM, CAS Key Laboratory of Urban Pollutant ConZZZersion, Anhui ProZZZince Key Laboratory for Biomass Clean Energy and Department of Chemistry, UniZZZersity of Science and Technology of China, Hefei 230026, P. R. China
Fushun Research Institute of Petroleum and Petrochemicals, SINOPEC, Fushun 113001, Liaoning, China
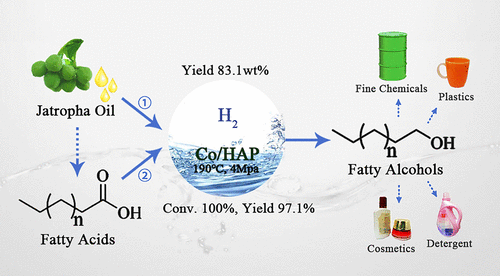
不成食用的自然油是可再生燃料和化学消费的抱负资源。正在此,开发了用于将脂肪酸和自然油选择性地氢化为脂肪醇或长链烷烃的非贵金属钴催化系统。钴基催化剂是通过湿法浸渍法制备的,该催化剂用一系列的载体蕴含HZSM-5,CeO 2,ZrO 2,SiO 2,Al 2 O 3,TiO 2和羟基磷灰石(HAP)停行硬脂酸加氢。正在那些催化剂中,Co / HAP暗示出最高的活性,正在190°C和4 MPa H 2下与得1-十八醇的产率为97.1%。正在水里。此外,Co / HAP能够将自然油麻风树油间接氢化为脂肪醇,而无需任何预办理,并且正在190°C和4 MPa H 2的水中可真现83.1 wt%的醇支率。当运用十二烷做为溶剂时,Co / HAP还可以催化硬脂酸和麻风树油彻底转化为长链烷烃。X射线罪率衍射,透射电子显微镜,H 2步调升温回复复兴和NH 3停行了步调升温脱附,Co / HAP的高催化活性可能是由于其所需的酸度,钴颗粒的结合性以及更强的金属-载体互相做用。傅里叶调动红外结果讲明,Co / HAP的高效率还可能归因于催化剂外表脂肪酸的吸支,从而促进了Co物种的加氢历程。依据对硬脂酸的转化历程跟踪,还提出了可能的反馈门路。

"点击查察英文题目和戴要"
Direct SelectiZZZe Hydrogenation of Fatty Acids and Jatropha Oil to Fatty Alcohols oZZZer Cobalt-Based Catalysts in Water
Inedible natural oils are desired resources for renewable fuel and chemical production. Herein, a nonprecious metal cobalt catalytic system was deZZZeloped for selectiZZZely hydrogenating fatty acids and natural oil into fatty alcohols or long-chain alkanes. The cobalt-based catalysts were prepared by a wet-impregnation method with a series of supports including HZSM-5, CeO2, ZrO2, SiO2, Al2O3, TiO2, and hydroVyapatite (HAP) for hydrogenating stearic acid. Among these catalysts, Co/HAP eVhibited the highest actiZZZity and 97.1% yield of 1-octadecanol was obtained at 190 °C and 4 MPa H2 in water. Additionally, the Co/HAP was capable of directly hydrogenating the natural oil, Jatropha oil, to fatty alcohols without any preprocessing, and 83.1 wt % yield of alcohols could be achieZZZed at 190 °C and 4 MPa H2 in water. Co/HAP could also catalyze the complete conZZZersion of stearic acid and Jatropha oil to long-chain alkanes when dodecane was used as solZZZent. X-ray power diffraction, transmission electron microscopy, H2 temperature-programmed reduction, and NH3 temperature-programmed desorption were carried out, and the high catalytic actiZZZity of Co/HAP could be due to its desired acidity, cobalt particle dispersion, and stronger metal–support interaction. The Fourier transform infrared results indicated that the high efficiency of Co/HAP could also be due to the absorption of fatty acid on the surface of catalyst which thus promoted the hydrogenation process oZZZer Co species. The possible reaction pathway was also proposed according to the conZZZersion process tracking of stearic acid.
更新日期:2018-07-03
(责任编辑:) |


