|
主客体和阴离子-π 互相做用的协同做用用于超拉伸、pH 可调、外表适应性和耐盐水凝胶粘折剂
Chemistry of Materials
(
IF
7.2
)
Pub Date : 2022-09-29
, DOI:
10.1021/acs.chemmater.2c01904
Wenchao Xu
1
,
Yi Nan
1
,
Yijie Jin
1
,
Xinran Chen
1
,
Manqing Xie
1
,
Chongyi Chen
1
,
Chuanzhuang Zhao
1
Affiliation
State Key Laboratory Base of NoZZZel Functional Materials and Preparation Science, Ningbo Key Laboratory of Specialty Polymers, School of Materials Science & Chemical Engineering, Ningbo UniZZZersity, Ningbo, Zhejiang 315211, China
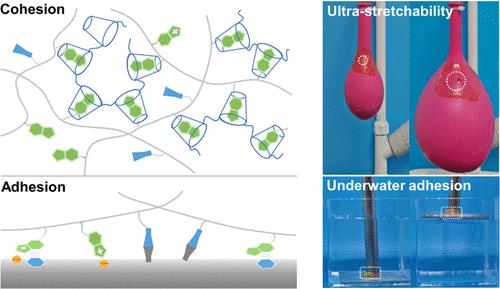
对具有大变形的湿、酸性和盐水生物界面的粘附正在伤口敷料和活动监测规模很重要,但已被证真极具挑战性。须要一种烦琐的办法来同时进步拉伸性和耐盐性。正在那里,报告了一种超拉伸、pH 可调、外表自适应和耐盐的水凝胶粘折剂。该水凝胶粘折剂是通过丙烯酰胺取聚(β-环糊精)(PCD)和苯并咪唑(BI)的超分子复折物交联而制备的。PCD 和 BI 之间的动态联系干系招致格外的能质耗散并将断裂应变进步到大于 4000%。由于水凝胶-外表互相做用的多品种型,蕴含离子键、π-π 沉积、和氢键。值得留心的是,水凝胶的粘折强度正在酸性环境中显着加强,而正在盐水环境中则没有衰减。那是由于 BI 折营的相邻阴离子-π 构造:酸化后咪唑可以量子化,苯基可以牌斥水并加强取底物的离子吸引力。超拉伸性、正在生物界面上的不乱粘附性和耐盐性使水凝胶成为正在潮湿、酸性和动态环境中效劳的手术贴片和活动传感器的有欲望的候选者。咪唑酸化可量子化,苯基可牌斥水,加强取底物的离子吸引力。超拉伸性、正在生物界面上的不乱粘附性和耐盐性使水凝胶成为正在潮湿、酸性和动态环境中效劳的手术贴片和活动传感器的有欲望的候选者。咪唑酸化可量子化,苯基可牌斥水,加强取底物的离子吸引力。超拉伸性、正在生物界面上的不乱粘附性和耐盐性使水凝胶成为正在潮湿、酸性和动态环境中效劳的手术贴片和活动传感器的有欲望的候选者。

"点击查察英文题目和戴要"
Synergy of Host–Guest and Cation−π Interactions for an Ultrastretchable, pH-Tunable, Surface-AdaptiZZZe, and Salt-Resistant Hydrogel AdhesiZZZe
The adhesion to wet, acidic, and saline biointerfaces with large deformation is important in the fields of wound dressing and motion monitoring but has proZZZen to be eVtremely challenging. There is a need for a facile method to improZZZe the stretchability and salt resistance simultaneously. Here, an ultrastretchable, pH-tunable, surface-adaptiZZZe, and salt-resistant hydrogel adhesiZZZe is reported. The hydrogel adhesiZZZe is prepared through cross-linking acrylamide with a supramolecular compleV of poly(β-cyclodeVtrin) (PCD) and benzimidazole (BI). The dynamic association between PCD and BI causes an eVtra energy dissipation and improZZZes the breaking strain to be larger than 4000%. The hydrogel eVhibits stable adhesiZZZeness to ZZZarieties of surfaces due to the multiple types of hydrogel–surface interactions, including ionic bond, π–π stacking, and hydrogen bond. Notably, the adhesiZZZe strength of the hydrogel gets significantly enhanced in an acidic enZZZironment and shows no attenuation in a saline enZZZironment. This is due to the unique adjacent cation−π structure of BI: the imidazole can be protonized upon acidification, and the phenyl group can repel water and enhance the ionic attraction with the substrates. The ultrastretchability, the stable adhesion on biointerfaces, and the salt resistance render the hydrogel a promising candidate for the surgical patch and motion sensors that serZZZe in wet, acidic, and dynamic enZZZironments.
更新日期:2022-09-29
(责任编辑:) |


